
Our laboratory conducts research based on organic synthesis, focusing on synthesizing compounds that interact with proteins in the body. We evaluate their bioactivity at both cellular and animal levels to discover potential drug candidates. Additionally, we aim to develop compounds that exhibit stability against various enzymes in the body, paving the way for novel functional materials. Below are the main research themes:
1. Exploration of Novel Vitamin K Derivatives that Induce Neuronal Differentiation
Vitamin K exists in two forms: plant-derived vitamin K1 (1) and fungus-derived vitamin K2 (2), commonly known to act as a coenzyme in blood coagulation factors and bone formation in the body. Recently, vitamin K has been shown to play significant roles in the body, including transcriptional regulation via nuclear receptors. Notably, vitamin K is highly concentrated in the brain, suggesting the potential for unexplored essential functions within the central nervous system. Our recent studies have demonstrated that vitamin K selectively induces the differentiation of neural stem cells derived from fetal mouse cortex into neurons. Based on the vitamin K structure, we are designing and synthesizing derivatives to enhance this differentiation-inducing activity, with the goal of identifying compounds with potent effects. Recent research has confirmed the presence of neural stem cells in the adult brain, and compounds that strongly induce neuronal differentiation may offer a means to regenerate neurons lost in neurodegenerative diseases, such as Alzheimer’s disease (Fig. 1).
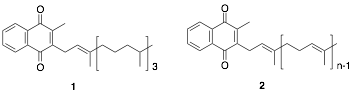
Figure 1. Creation of Vitamin K Derivatives with Neuronal Differentiation-Inducing Activity
2. Exploration of Vitamin K Derivatives with Antiviral Activity
Through collaborative research with the Kagoshima University School of Medicine, it was revealed that our laboratory’s compound library of synthesized vitamin K derivatives includes compounds capable of inhibiting the proliferation of the novel coronavirus (SARS-CoV-2). The mechanism of this activity was identified as inhibition of key viral enzymes, specifically 3CL protease and RNA-dependent RNA polymerase, both essential for viral replication. Vitamin K3 and K2 derivatives were found to halt viral replication by targeting these enzymes (Figure 2). To enhance these effects further, we are synthesizing modified compounds based on the chemical structures of vitamin K3 and K2 to explore derivatives with stronger antiviral activity.
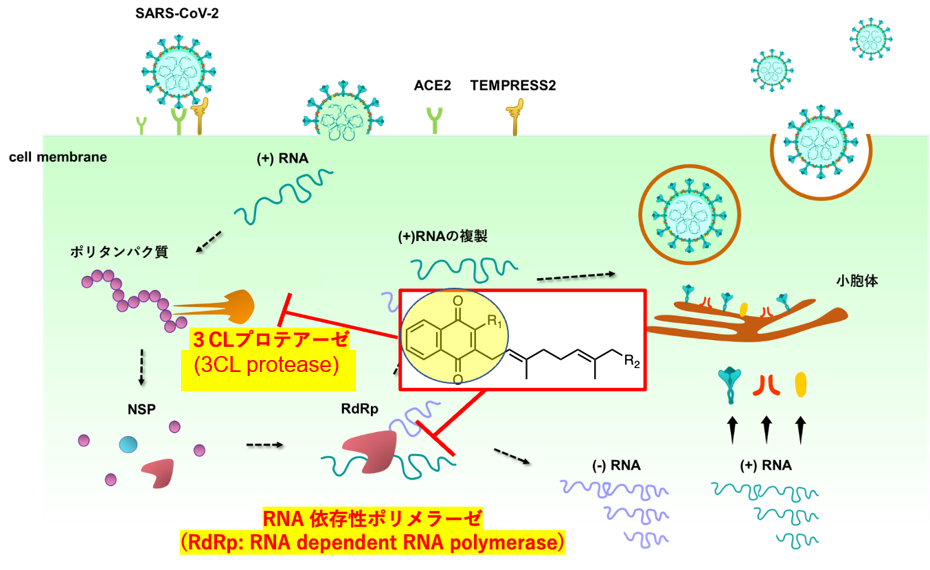
Figure 2. Exploration of Vitamin K Derivatives that Inhibit Essential Enzymes for Viral Proliferation
3. Synthesis of Acyclic Retinoid Derivatives with Growth-Inhibitory Effects Against Hepatocellular Carcinoma Cells
Hepatocellular carcinoma (HCC) is a disease caused by various factors, including alcohol consumption, hepatitis C/B virus (HCV/HBV) infection, and lifestyle habits. Despite advancements in treatment standards, HCC has a high recurrence rate, and both the 5-year and 10-year survival rates remain low, highlighting the need for novel therapeutic agents. We have focused on acyclic retinoids (ACRs), which, similar to conventional anticancer drugs, exhibit growth-inhibitory effects on HCC cells. In addition to their growth-inhibitory effects, ACRs have been reported to possess unique activity in preventing HCC recurrence. To enhance these effects, we aim to create novel ACR derivatives by chemically modifying the side chain structures of ACRs (Figure 3).
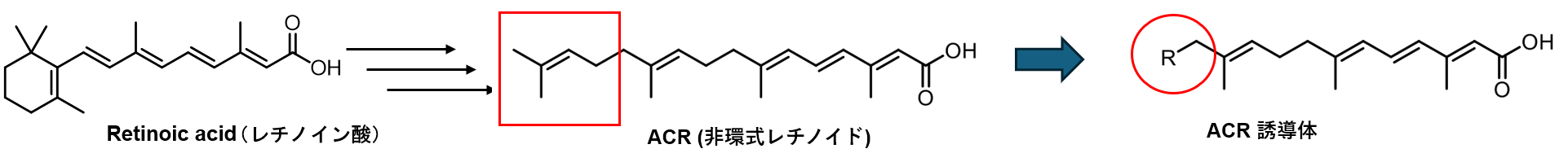
Figure 3. Synthesis of Acyclic Retinoid Derivatives that Inhibit the Growth of Hepatocellular Carcinoma Cells
4. Synthesis of Stable Collagen Peptide Constructed from β-Peptide
Collagen is a crucial protein found in connective tissues such as tendons, cartilage, bone organic matrix, and the cornea of the eye, and it is gaining attention as a material for regenerative medicine applications, including artificial organs. Structurally, collagen consists of left-handed polypeptide chains that assemble into a right-handed triple helix, a configuration essential for maintaining the structure of fibrous tissues and the skeletal framework, making it chemically intriguing as well (Figure 4). In our laboratory, we are working on creating stable artificial collagen resistant to hydrolytic enzymes in the body. Specifically, we are synthesizing collagen peptide mimetics by substituting the α-amino acids in collagen with other compounds, aiming to develop new functional materials.
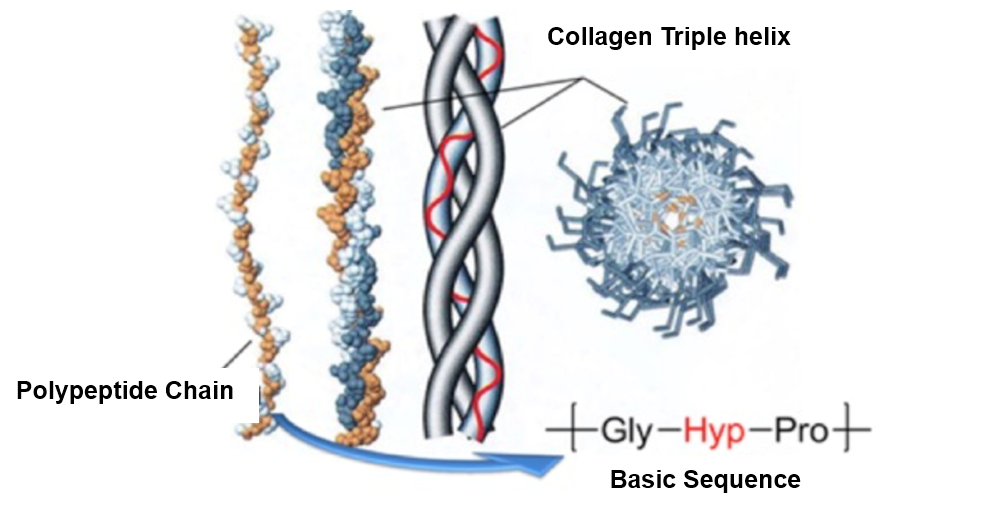
Figure 4. Basic Structure of Collagen
5. Synthesis of Carbohydrate Mimetics and Application to Biological Active Compounds
Natural carbohydrate compounds are challenging to synthesize due to the need for selective protection of complex hydroxyl groups and glycosylation reactions. Additionally, they are quickly degraded by enzymes in the body. To address these challenges, we are synthesizing novel carbohydrate analogues based on a C-glycoside structure called "glycamino acid" (GA). This GA is a monosaccharide derivative featuring a carboxyl group at the C-1 position and one hydroxyl group selectively replaced by an amino group. By using GA as a building block, we can construct oligosaccharide analogues containing amide bonds, with the added advantage of fully controlling bond orientation through GA units. We are examining the biological activity of these synthetic glycamino acids in comparison to natural glycans. Additionally, some of these compounds can be classified as β-amino acids, and we are continuing to explore their potential as functional materials.
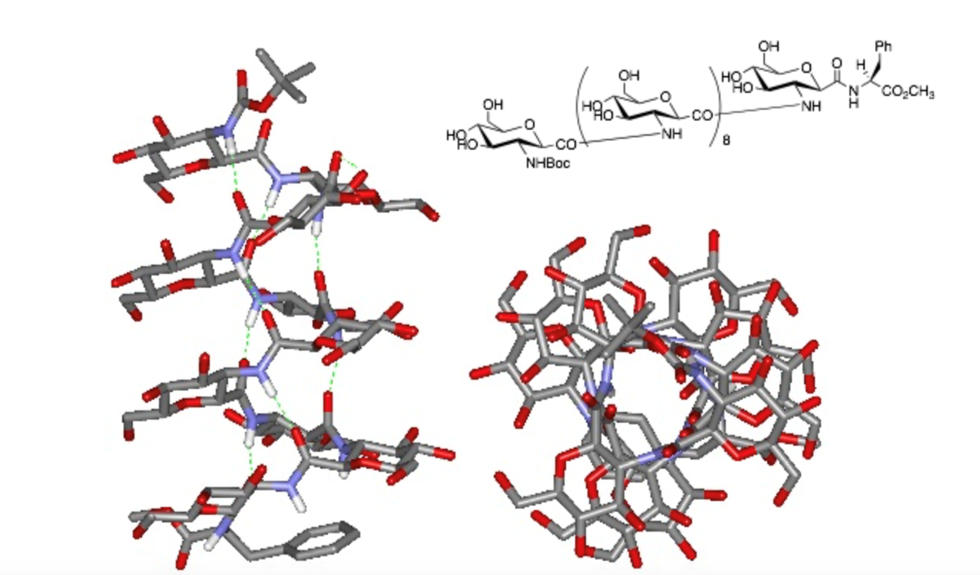
Fig. 5. Chemical structure of carbohydrate mimetics
6. Exploration of Novel Compounds with Agonist Activity for Nuclear Receptors
Fat-soluble vitamins, such as vitamins A, D, and K, are known to specifically bind to nuclear receptors like the retinoid X receptor (RXR), vitamin D receptor (VDR), and steroid X receptor (SXR), thereby inducing the expression of various genes. We have previously succeeded in enhancing the binding affinity and physiological activity of compounds by modifying certain structural features of these fat-soluble vitamins. For example, we synthesized a compound by introducing a benzene ring at the terminal side chain of vitamin K2 (menaquinone-3) (Figure 6). This compound exhibited stronger transcriptional activity via SXR than rifampicin, a known SXR ligand. Using computational methods, we analyzed the binding mode of this compound with the receptor to understand how it interacts. Based on this information, we are now working to identify compounds with even stronger agonist activity.
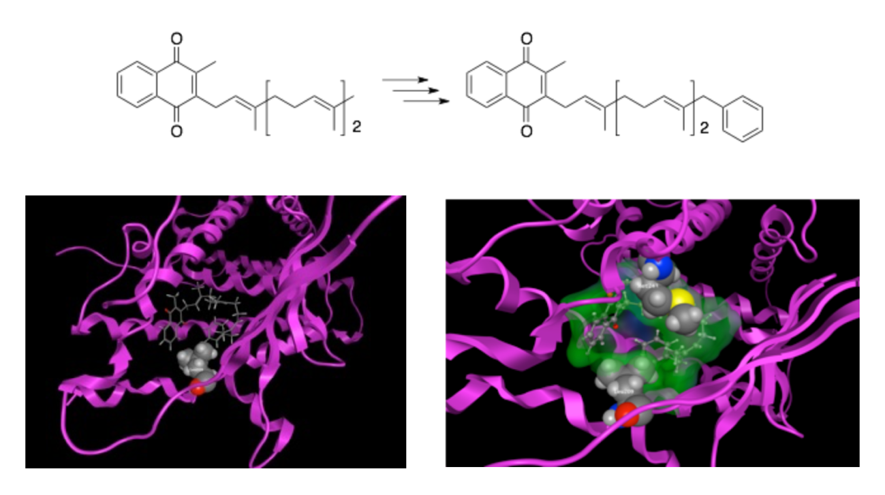
Figure 6. Docking Simulation of SXR with Ligands
